Research activities of Laboratory MMM
MAX-phase materials (Mn+1AXn, n = 1, 2 or 3) [1] are a family of nano-layered hexagonal compounds. In these materials, M is an early transition metal, A is an element of the main group, and X is C or N (n = 1-3). These systems have an atomic-layered structure (figure 1 and figure 2) consisting of m-XM (M2X) layers alternating with the atomic layers of the A-element. The atomic layers are stacked along the C axis. The layered highly anisotropic crystal structure leads to mechanical properties usually associated with ceramics. MAX-phases combine both ceramic and metal characteristics, providing resistance to high-temperature oxidation, self-healing ability and resistance to thermal shock. Such outstanding mechanical properties have made them interesting materials, for example, for treatable, heat-resistant refractories, heating elements or coatings for electrical contacts. In terms of applications, MAX-phase materials have shown very promising properties for batteries and ultra-high-frequency devices [2]. In addition, MAX-phase materials serve as "raw materials" for the synthesis of delaminated two-dimensional transition metal carbides (MXenes) [3].
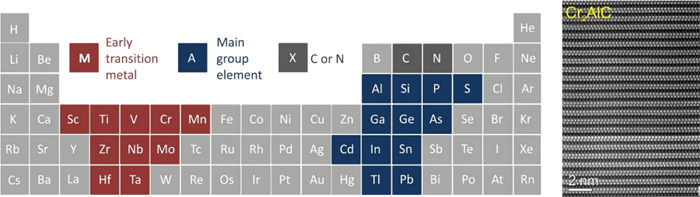
Figure 1-Periodic table of elements with marked elements that form known MAX-phase phases (red = M, blue = A, grey = X), adapted from http://max.materials.drexel.edu (left). Atomic structure of MAX phase Cr2AlC (right).
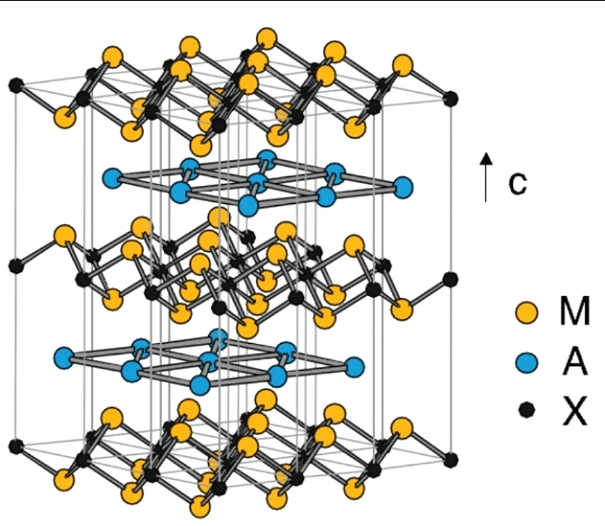
Figure 2-Nano-layered structure of the Mn+1 An phase for n = 1. The hexagonal connections form MXM sheets separated by layers A.
It has long been believed (since the 1960s) that the layered structure of MAX can only be obtained by using early transition metals as the main component of the m layers, which essentially leads to the formation of only paramagnetic compounds. The first MAX phase (Cr0.75Mn0.25)2GeC with long-range magnetic order was synthesised as an epitaxial thin film on MgO (111) in 2013 [4]. Subsequent studies have found several triple and quarter MAX-phases based on Mn with competing ferro - and antiferromagnetic (intra - and interplane) interactions leading to a common complex magnetic response, depending on the field and temperature. Recently, a detailed study of the magnetic properties of Mn2GaC led to the discovery of the first magneto-caloric MAX-phase with a high ordering temperature and inversion of the magnetostriction and magnetoresistance sign at the phase transition [5]. These new material properties offer new functionality for smart sensors and actuators that are in demand for the “Internet of things”.
Despite the abundance of work on the synthesis and research of thin films of MAX-phases, the systematic study of MAX materials demonstrating ferromagnetic properties is still in its infancy [5]. Since MagMAX materials can contain 4 or more elements, a combination of high-performance deposition [6] and detailed structural, compositional, magnetic and spectroscopic analysis methods is required to find new compounds and the best composition. In addition, the problem of the crystalline quality of the films, as well as the presence of accompanying phases of carbides and intermetallics, remains unsolved.
Most of the first work on the synthesis of thin films of MAX-phases was performed using physical vapour deposition from the gas phase, mainly by magnetron sputtering, as well as using cathode-arc evaporation [7]. The synthesis of thin MAX-phase films using sputtering methods can be divided into three main approaches: joint sputtering with three elementary targets, sputtering with complex targets, and solid-phase synthesis of reactions by spraying an amorphous multilayer material [7]. Sputtering from M, A, and graphite targets is the most common method for laboratory synthesis of MAX carbide thin films. The main advantage of using three elementary targets is flexibility in individual control of element flows [7]. The initial values of the deposition parameters are based on some estimates or calibrations, and then the usual optimisation procedure is performed to achieve the desired MAX phase of stoichiometry [8]. Using this approach, the synthesis of thin films of different phases was successfully demonstrated [9, 10]. Reactive spray deposition has been relatively little studied for the synthesis of carbides and nitrides of MACH phases, mainly due to the fact that the technological interval (relative to the partial pressure of the chemically active gas) for the deposition of single-phase or high-purity MACH phases is extremely narrow [7]. Sputtering from complex targets is sometimes preferable for reasons of simplicity and reproducibility [11]. However, the general problem remains that the composition of the film can be very different from the nominal composition of the target.
Solid-state reaction synthesis can be used to deposit a multicomponent film in a metastable state, such as an amorphous multilayer structure containing three or more elements M, A and X in the corresponding composition, at normal or low temperatures [11]. After the application of the films, annealing at a certain temperature should be performed to cause the transformation into the MAX phase. Examples include annealing the magnetron sputtering of multilayer Ti / Si / C [12] to the corresponding MAX phases of Ti3SiC2.
In addition to the chemical composition in thin films, another important parameter for the growth of MAX-phase films is the choice of the substrate temperature or annealing temperature. The origin of the MAX phase structure requires significant thermal activation to ensure sufficient mobility and energy of the atoms for the desired arrangement of the atoms [7]. Lower deposition temperatures usually lead to the formation of corresponding carbides and / or intermetallic phases. In addition, more recent studies have also shown that thin films of different phases can be stabilised directly on substrates with a sufficiently high lattice mismatch on a single-crystal substrate [7].
- Sokol, V. Natu, S. Kota and M. W. Barsoum, Trends in Chemistry 1 (2), 210-223 (2019).
- M. R. Lukatskaya, S. Kota, Z. Lin, M.-Q. Zhao, N. Shpigel, M. D. Levi, J. Halim, P.-L. Taberna, M. W. Barsoum, P. Simon and Y. Gogotsi, Nature Energy 2, 17105 (2017).
- N. K. Chaudhari, H. Jin, B. Kim, D. San Baek, S. H. Joo and K. Lee, J Mater Chem A 5 (47), 24564-24579 (2017).
- R. Salikhov, A. S. Semisalova, A. Petruhins, A. S. Ingason, J. Rosen, U. Wiedwald and M. Farle, Materials Research Letters 3 (3), 156-160 (2015). 5. F. M. Römer, U. Wiedwald, T. Strusch, J. Halim, E. Mayerberger, M. W. Barsoum and M. Farle, RSC Advances 7 (22), 13097-13103 (2017).
- P. Eklund, M. Beckers, U. Jansson, H. Högberg, L. Hultman, The MN+1AXN phases: Materials science and thin-film processing, Thin Solid Films. 518 (2010) 1851–1878
- A.S. Ingason, A. Petruhins, J. Rosen, Toward Structural Optimization of MAX Phases as Epitaxial Thin Films, Mater. Res. Lett. 4 (2016) 152–160.
- O. Wilhelmsson, J.-P. Palmquist, T. Nyberg, U. Jansson, Deposition of Ti2AlC and Ti3AlC2 epitaxial films by magnetron sputtering, Appl. Phys. Lett. 85 (2004) 1066–1068
- O. Wilhelmsson, J.-P. Palmquist, E. Lewin, J. Emmerlich, P. Eklund, P.O.Å. Persson, H. Högberg, S. Li, R. Ahuja, O. Eriksson, L. Hultman, U. Jansson, Deposition and characterisation of ternary thin films within the Ti–Al–C system by DC magnetron sputtering, J. Cryst. Growth. 291 (2006) 290–300.
- D.E. Hajas, M. to Baben, B. Hallstedt, R. Iskandar, J. Mayer, J.M. Schneider, Oxidation of Cr2AlC coatings in the temperature range of 1230 to 1410°C, Surf. Coat. Technol. 206 (2011) 591–598.
- I.C. Schramm, C. Pauly, M.P. Johansson Jõesaar, P. Eklund, J. Schmauch, F. Mücklich, M. Odén,165 Solid state formation of Ti4AlN3 in cathodic arc deposited (Ti1-xAlx)y alloys, Acta Mater. 129 (2017) 268–277
- M. Hopfeld, R. Grieseler, T. Kups, M. Wilke, P. Schaaf, Thin film synthesis of Ti3SiC2 by rapid thermal processing of magnetron-sputtered Ti-C-Si multilayer systems, Adv. Eng. Mater. 15 (2013) 269–275.
